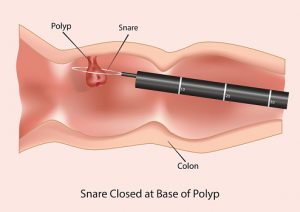
A standard colonoscopy requires the insertion of a flexible tube with a camera at the end into the rectum. A doctor will guide the tube through the colon and check the patient for growths, known as polyps, which if left unchecked can develop into colon cancer. This procedure occurs while the patient is under sedation and allows the physician to remove any polyps present.
While the standard colonoscopy is effective in finding and removing polyps as well as screening for colon cancer, some patients are unable to tolerate the procedure or the sedation necessary. In this case, technology such as the capsule—which can also provide visuals of any polyps in the digestive tract—could prove beneficial as it is noninvasive and allows for the patient to be screened without the use of anesthesia. The patient simply swallows the capsule with water, and once it is excreted and flushed, returns the belt to their physician who can then examine the collected images for any evidence of polyps. If a polyp is found, the patient will have to undergo a traditional colonoscopy to remove it.
This new technology has the potential to spare patients who have difficulty tolerating colonoscopies the invasive procedure. The capsule may also increase the likelihood of catching cases of colorectal cancer earlier, as it is more convenient for patients and less intimidating.
Currently, the FDA has approved the use of the capsule imaging system in patients who experience lower gastrointestinal bleeding but are not suitable for a colonoscopy due to age or other risk factors, and for those whose colon anatomy makes performing the procedure too difficult.